Taking one for the team: 6 ways our cells can die and help fight infectious disease
- Written by Georgia Atkin-Smith, Research scientist, La Trobe University
We have all heard of COVID-19, the flu and bacterial infections. But what is actually happening to our cells when we contract these diseases? Many of our body’s cells don’t live to tell the tale. But cell death isn’t necessarily a bad thing — in fact, the death of infected cells can provide a sacrificial mechanism to stop pathogens in their tracks before they can spread through our body.
Over the years, researchers have realised there are many ways for our cells to die. Our genetics contain a comprehensive “licence to die”, with the route to cell death dictated by both the type of the cell and the pathogen. Let’s check some out:
The dancing death
In the time it takes you to read this sentence, ten million cells in your body will have died, through a type of death called apoptosis. This term, coined in 1972 by Australian pathologist John Kerr, comes from the Greek phrase for “leaves falling from a tree”.
Apoptosis is the most common form of cell death, and has also been nicknamed the “dance of death”, because of the extraordinary shape changes exhibited by the cells under a microscope as they sacrifice themselves.
For example, apoptotic cells dying from radiation or infection with influenza A virus (aka, the flu) generate large, bubble-like structures on their surface called blebs, before shooting out long beaded necklace-like protrusions and finally shattering into pieces.
The death of flu-infected cells is suggested to both aid and limit viral spread. Nevertheless, it’s a spectacular event to witness (and an excellent reminder to get your flu shot this winter).
White blood cell blebbing and dying.Out with a bang
Vaccinia virus is used worldwide to vaccinate against smallpox. In fact, it was the very first vaccine, developed in 1796 by Edward Jenner.
We now also know that vaccinia virus can make our cells more sensitive to a particular type of cell death, caused by a molecule called TNF. This can help prevent the disease spreading by killing off infected cells before the virus has a chance to replicate.
Many of our cells have a roughly spherical or balloon-like shape, encapsulated by a protective layer called the cell membrane. Just like bursting a balloon with a pin, puncture to the cell membrane marks the point of no return.
This process occurs during necroptosis — an explosive type of cell death in which proteins inside the cell punch holes in the membrane. The cell pops and dies, shutting down the machinery needed for viral replication.
The spider web of death
When they aren’t busy haunting our nightmares, spiders can be found weaving silken masterpieces of extraordinary detail and strength. The web of a golden orb weaving spider, for example, is strong enough to entangle small birds.
On a smaller but equally impressive scale, our immune system contains specialised cells called neutrophils that can weave a deadly web of their own and entrap bacteria. Neutrophils gallantly sacrifice themselves in the process of casting their web, in a type of cell death perhaps fittingly called NETosis.
When infected with bacteria such as Streptococcus pneumoniae, which causes pneumonia and meningitis, neutrophils eject a specialised web made from their own DNA. These webs can entangle nearby bacteria to prevent their escape until other immune cell reinforcements arrive to clear the infection. Sometimes, proteins found in these webs can also kill the bacteria - quite an impressive defence mechanism!
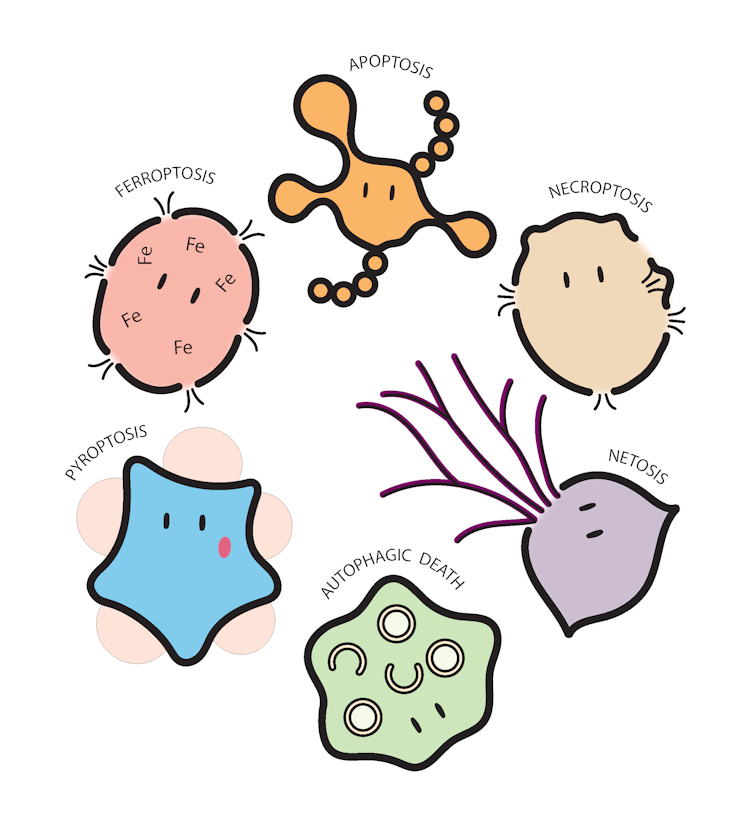 There are a surprising number of ways cells can lay down their lives for the greater good.
Author provided
There are a surprising number of ways cells can lay down their lives for the greater good.
Author provided
The last meal
Just as our bodies are compartmentalised into organs such as the stomach, liver or heart, our individual cells also have specialised compartments. One of the cell’s “stomachs” (a structure called the “autophagosome”) engulfs and digests cellular contents such as damaged molecules through the process of autophagy.
However, in some circumstances, the machinery that drives this Pac-Man-style action can also facilitate the cell’s demise. Coincidentally, the bacteria Helicobacter pylori can infect cells of the human stomach lining, called epithelial cells, which can cause ulcers and gastritis. The cells can respond with a process called autophagic cell death, in which the induction of autophagy causes the cell to die.
A fiery death
Pyromania, derived from the Greek word pyr, meaning fire, is an obsessive desire to set things ablaze. Some of our immune cells also have the ability to self-immolate and cause inflammation as part of our response to infection.
Since its relatively recent discovery in 2001, this type of cell death, called pyroptosis, has become a hot topic (sorry) among cell biologists, and is often facilitated by a molecular complex called the inflammasome.
Read more: What is autoinflammatory disease, the rare immune condition with waves of fever?
In 2021, understanding pyroptosis is more important than ever, as it has been linked to infection with SARS-CoV-2 infection, the virus that causes COVID-19.
Activation of the factors that cause pyroptosis may help explain the excessive inflammation seen in patients with severe COVID-19. And this could potentially offer a new way to combat the disease.
Overdosing on iron and fat
There’s no doubt the key to a long and healthy life is a balanced diet and exercise. However, sometimes we can’t resist the urge to devour a burger and fries with ice cream for dessert. With enough hard work, we can burn it off again. But for individual cells, overindulging can be fatal.
Too much iron and/or harmful types of fat molecules can cause cells to die by ferroptosis. Cells infected with Mycobacterium tuberculosis, the bacterium that causes TB, can increase their iron content and cause ferrototic cell death! Pass the salad, thanks.
Read more: Tick, tock... how stress speeds up your chromosomes' ageing clock
The survival of the human body is a fine balancing act between cell growth and cell death. Understanding our cells’ complex “licence to die” could give us new ways to combat disease.
Authors: Georgia Atkin-Smith, Research scientist, La Trobe University





