Life frozen in time under an electron microscope gets a Nobel Prize
- Written by Xavier Conlan, Senior Lecturer Forensic Chemistry, Deakin University
The scientists who developed the ability to see some of the building blocks of life under the electron microscope have been awarded the 2017 Nobel Prize in Chemistry.
Jacques Dubochet, Joachim Frank and Richard Henderson pioneered cryo-electron microscopy, which the Royal Swedish Academy of Sciences said both simplifies and improves the imaging of biological molecules, known as biomolecules.
The 9 million Swedish kronor (A$1.4 million) prize is split equally between Dubochet, at Switzerland’s University of Lausanne, Frank, at New York’s Columbia University, and Henderson, at the MRC Laboratory of Molecular Biology, Cambridge in the UK.
 The 2017 winners of the Nobel Prize in Chemistry: (left to right) Joachim Frank, Richard Henderson and Jacques Dubochet.
Reuters/Brendan McDermid, Toby Melville and Denis Balibouse
The 2017 winners of the Nobel Prize in Chemistry: (left to right) Joachim Frank, Richard Henderson and Jacques Dubochet.
Reuters/Brendan McDermid, Toby Melville and Denis Balibouse
The Academy said the method developed by the three researchers had moved biochemistry into a new era. The technology now allows researchers to generate a high resolution view of biomolecules while they exist in their natural state.
Read more: An award with real gravity: how gravitational waves attracted a Nobel Prize
The biological lock and key
The human body is amazingly complex and requires the cooperation of a range of biochemical mechanisms, such as digestion and energy production, in order to function well. These intricate processes involve the use of biomolecules, typically large entities made from amino acids – the building blocks of life.
Importantly, just like the construction of any brick-built house, the configuration or placement of the blocks is critical to how well our construction stands up, or how well our biomolecules function.
Furthermore, biomolecules present their capacity to perform tasks by interacting with other entities, such enzymes, in the body. These are based on a specific configuration, much like how only one key can open a particular lock.
The significant challenge overcome by the award-winning team was to develop the capacity to observe the biomolecules in their natural state. Before the advent of cryo-electron microscopy, they were visualised with X-Ray crystallography.
It was also thought that electron microscopes were only suitable for imaging dead matter, because the powerful electron beam destroys biological material.
The key breakthrough came with the development of a process to rapidly freeze a sample. This enabled the biomolecules to be captured in their bespoke configuration.
The team identified early on in their work that freezing a sample prior to visualisation may afford the improvement required to fully interrogate the biomolecules.
Frozen in time
Here is where the fun starts. While sounding inherently simple, rapidly freezing a sample is particularly challenging.
If the process removes the water from the sample then the biomolecule collapses, losing the natural configuration desired by the researchers. If the sample is frozen too slowly then ice crystals form, which also interferes with the biomolecule’s configuration.
The team developed a process known as vitrification. This freezes the sample at -190℃ while it is placed on a wire mesh, an elegantly simple approach to solving a difficult problem.
Like most Nobel prizewinning scientific achievements, the development was incremental. Changes by the team over many years enabled the combination of the freezing process (developed in 1978) and the microscopy technology which was only fully realised in 2013.
This combination and advancement in technology enabled the high-resolution imaging of biomolecules.
Unlocking a virus
So what does all this mean? Well, understanding the configuration of the lock enables scientists to cut a particular key.
Viruses are large biomolecules. Once visualised, scientists can identify molecules or develop pharmaceutical keys that can fit into their structure in order to break them apart or disrupt their function.
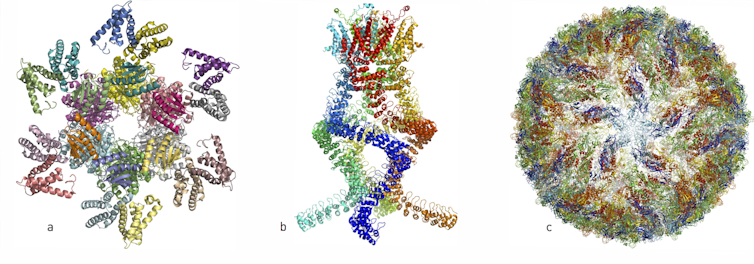 Over the past few years, researchers have published atomic structures of numerous complicated protein complexes: (left, a) a protein complex that governs the circadian rhythm, (centre, b) a sensor of the type that reads pressure changes in the ear and allows us to hear, and (right, c) the Zika virus.
The Royal Swedish Academy of Sciences
Over the past few years, researchers have published atomic structures of numerous complicated protein complexes: (left, a) a protein complex that governs the circadian rhythm, (centre, b) a sensor of the type that reads pressure changes in the ear and allows us to hear, and (right, c) the Zika virus.
The Royal Swedish Academy of Sciences
An example of the power of cryo-electron microscopy is seen through the rapid characterisation of the Zika virus soon after it was first identified as a major global health risk.
The identification of the configuration of the virus and the pocket of the biomolecule that joins to its host will form the basis for ongoing studies on how best to combat this virus.
Read more: Error correcting the things that go wrong at the quantum computing scale
The technology has also had an impact at the dinner table. A US research team has investigated the heat-sensing component of the tongue, highlighting the wasabi sensor. This may offer the potential to better understand new pain-management approaches.
In Australia, a consortium is exploiting the power of this technology to probe diseases related to the immune system in order to develop better treatment protocols.
Cryo-electron microscopy will be an exciting area to watch in the near future, for locksmiths and science enthusiasts alike.
Authors: Xavier Conlan, Senior Lecturer Forensic Chemistry, Deakin University
Read more http://theconversation.com/life-frozen-in-time-under-an-electron-microscope-gets-a-nobel-prize-85246




