Vaccine progress report: the projects bidding to win the race for a COVID-19 vaccine
- Written by Liam Petterson, Assistant Editor, Health + Medicine, The Conversation Australia
The race is on to develop a vaccine for COVID-19. There are now more than 140 vaccines being tested around the world, according to the World Health Organisation.
Australian researchers are leading several major clinical trials that might help bring an end to the deadly disease.
We asked the lead investigators of trials around Australia to tell us about their vaccines: when the trials are being held, how they work, and how they might kill or impede the SARS-CoV-2 coronavirus.
What you need to know
Kylie Quinn, RMIT
Vaccine development normally progresses through a series of steps that enable researchers to collect all the data needed to approve a vaccine for the clinic. Initially, vaccines are tested in the lab, using cells in culture and animals that mimic human disease, to determine whether the vaccine is safe and worth developing further.
Promising vaccines are then evaluated in clinical trials with human volunteers, where researchers ask:
Is the vaccine safe, and what dose should be used? (Phase 1)
Can the vaccine generate an immune response? (Phase 2)
Can the vaccine protect from infection or disease? (Phase 3)
With SARS-CoV-2, researchers are speeding up this process by running one trial while simultaneously recruiting for the next phase. This uses lessons learnt during the Ebola epidemic, when a vaccine was developed in a record time of just five years.
Despite this acceleration, many researchers’ best guess is that it will be at least early 2021 before a vaccine might be approved (and the next challenge is to manufacture enough of it). This may seem like a long time, but it is lightning-fast for a vaccine.
But it isn’t a sure thing yet. One concern is that when people recover from infection with other human coronaviruses, immunity can wane relatively quickly. It seems vaccines will have to do much better than this “natural immunity”.
Fortunately, early data from human trials suggests vaccines can generate stronger responses than natural immunity. Our next questions are: how long will these responses last, and will they protect us?
The waiting game continues.
Read more: Immunity to COVID-19 may not last. This threatens a vaccine and herd immunity
Here are some of the leading vaccine candidates so far:
‘Molecular clamp’ vaccine (University of Queensland)
Professor Paul Young, University of Queensland
The University of Queensland team began developing a vaccine against COVID-19 on January 10 – the day the genetic sequence of the SARS-CoV-2 virus was first made public. We didn’t need access to the virus itself, just details of its genetic sequence, from which we identified the gene that encodes the “spike protein” used by the virus to infect human cells.
Read more: Revealed: the protein 'spike' that lets the 2019-nCoV coronavirus pierce and invade human cells
As part of our rapid response vaccine program we were able to engineer this protein to include the “molecular clamp”, a UQ technology that is designed to hold the protein in the form that appears on the surface of the virus, with the aim to make a better vaccine by design. This clamped version of the spike protein can then be manufactured using established methods from the biotechnology industry. We also think this is a potential advantage of our platform: while it takes slightly longer to get out of the blocks, the path for mass production exists with existing global expertise and infrastructure.
The manufactured protein is also combined with an adjuvant – an ingredient that helps stimulate a stronger immune response, enabling efficient protection from the virus. We are using technology from CSL/Seqiris which is used in vaccines for influenza and has an excellent track record of patient safety.
We have spent the past few months carrying out crucial preclinical work in animals, confirming the vaccine induces this strong immune response, is safe, and is able to protect animals from challenge with the live virus. We have also been developing the processes for manufacturing the vaccine at large scale with our commercial partner CSL.
Now these studies have been carried out successfully, we have begun clinical trials in humans. The phase 1 trial began on July 13 with volunteers receiving the first dose. The trial will involve a total of 120 volunteers. We will monitor safety and immune responses in these individuals and should have preliminary data in the few months. If this first trial is successful, the next phase of clinical trials will be conducted over the remainder of 2020 and early 2021 to determine how effective the vaccine is at preventing virus infection.
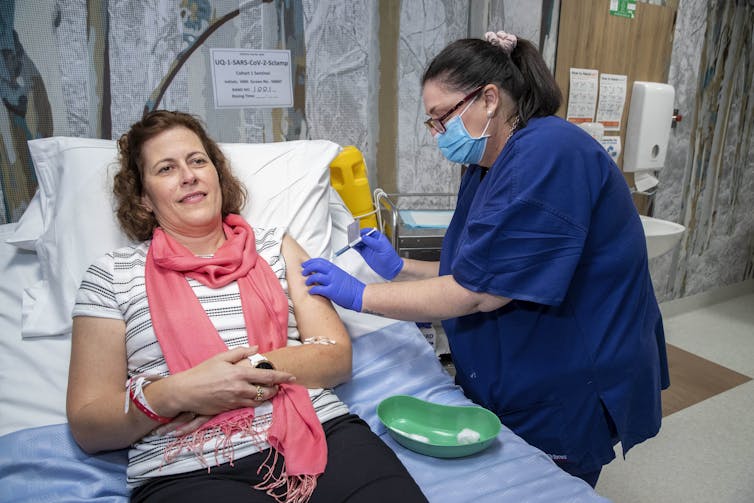 A volunteer in the University of Queensland’s phase 1 trials of it’s COVID-19 vaccine candidate.
Glenn Hunt/AAP
A volunteer in the University of Queensland’s phase 1 trials of it’s COVID-19 vaccine candidate.
Glenn Hunt/AAP
Novavax vaccine (tested in Melbourne and Brisbane)
Professor Paul Griffin, University of Queensland
In late May, US biotechnology company Novavax began phase 1 human trials of its COVID-19 vaccine candidate via Nucleus Network, a clinical research organisation. The trials, in Melbourne and Brisbane, involve about 130 healthy volunteers aged 18-59.
Novavax has a history of making protein-based vaccines for a number of other infections. What this means is they manufacture the antigen from scratch in the laboratory to resemble part of the surface of the infectious agent. This then hopefully generates an immune response in humans that will protect from infection without the need to be exposed to any actual virus.
Like several other current vaccine candidates, this trial vaccine is designed to trigger an immune response by mimicking the SARS-CoV-2 spike protein. Given this protein is found on the surface of the virus and is used to invade human cells, an immune response against it should help to prevent infection.
Novavax said it expects preliminary results to show how safe it is and how well it works in July 2020. The phase 2 portion of the study will begin promptly after phase 1, assuming it is successful.
BCG vaccine (tested by Murdoch Children’s Research Institute)
Professor Nigel Curtis, MCRI
The Bacillus Calmette-Guérin (BCG) vaccine has been around for nearly 100 years and is designed to protect against tuberculosis.
Read more: Could BCG, a 100-year-old vaccine for tuberculosis, protect against coronavirus?
However, the BCG vaccine also has “off-target” effects on the immune system which provide protection against a range of other infections. This happens because BCG elicits something called “trained immunity” — a boosting of the innate immune system, the body’s first line of defence.
Previous small trials suggest BCG vaccination might protect against respiratory infections in general, not just tuberculosis. In Guinea-Bissau, BCG vaccination reduced neonatal deaths from all causes by 38%. In South Africa, BCG vaccination reduced respiratory infections in adolescents by 73%. Therefore, BCG vaccination might provide a means of protecting health-care workers and other vulnerable individuals from COVID-19.
As reported in The Lancet, our team at the Murdoch Children’s Research Institute is conducting trials to assess whether BCG vaccination reduces the rate or severity of COVID-19 in health-care workers. We are currently recruiting 10,000 health-care workers in Australia, Europe and Latin America.
BCG vaccination is not going to be a silver bullet that provides perfect protection against COVID-19 or any other pandemic illness. However, we may find it provides some level of protection. If so, it will be a readily available to bridge the gap and protect health-care workers and other vulnerable individuals until a specific COVID-19 vaccine is developed.
The CSIRO is testing two vaccines
Professor S.S. Vasan, CSIRO
In 2019, CSIRO scientists at the Australian Centre for Disease Preparedness had been preparing for the next major epidemic dubbed “Disease X”. We were developing animal challenge models for rapid evaluation of candidate vaccines and therapies.
When Disease X turned out to be COVID-19, we were the first to show ferrets were susceptible to SARS-CoV-2, and used this disease model for preclinical evaluation of two vaccine candidates selected by the global Coalition for Epidemic Preparedness Innovations.
The first candidate is from the University of Oxford. Oxford scientists have inserted the SARS-CoV-2 genome into a defective adenovirus, which can begin an infection in human cells but cannot replicate to grow the infection. As the key coronavirus proteins are expressed, immunity is developed against future SARS-CoV-2 infection.
We are evaluating this vaccine as an injection into the muscles, as envisaged by the University. At CSIRO, we are also exploring whether delivering through a nasal spray could confer better protection against COVID-19.
 The CSIRO’s Australian Centre for Disease Preparedness in Geelong, Victoria. Researchers are testing two potential vaccines for SARS-CoV-2, the virus that causes COVID-19.
EPA/CSIRO/AAP
The CSIRO’s Australian Centre for Disease Preparedness in Geelong, Victoria. Researchers are testing two potential vaccines for SARS-CoV-2, the virus that causes COVID-19.
EPA/CSIRO/AAP
The second vaccine candidate is from a US company, Inovio Pharmaceuticals. It is an exciting new technology – no vaccine of this type has been approved for human use yet.
It consists of a small circle of DNA containing the genetic code for a particular protein from SARS-CoV-2. When it is administered as an injection, using a special “electrophoresis” device, it stimulates our cells to produce this protein themselves, meaning our body can then produce antibodies that will help prevent the actual virus from infecting our cells.
We are now at the final stages of the study. Our team is analysing very large amounts of data and samples generated by the preclinical trials. The results will be shared with regulators and through peer-reviewed publications following internal and external review, quality assurance and a compliance audit.
We have also been keeping track of how this virus is mutating, to ensure that the vaccines and the animal models used to evaluate them, are not impacted.
Vaxine and Flinders University researchers
Professor Nikolai Petrovsky, Flinders University
Alongside researchers from Flinders University, Australian biotechnology company Vaxine has created a vaccine aimed at protecting against SARS-CoV-2 infection. The vaccine, called “COVAX-19”, is based on a synthetic protein produced by growing it in insect cells which is designed to mimic the spike protein on the outside of the SARS-CoV-2 virus that it uses to attach to human cells.
The vaccine was based on the company’s earlier SARS-1 coronavirus vaccine that successfully protected against infection in animal models and also induced strong antibody and T cell responses against COVID-19 in animals (as we explore in studies soon to be submitted for publication).
The vaccine entered human clinical trials on July 1, being the first Australian-developed vaccine to achieve this milestone. The phase 1 trial is being performed at the Royal Adelaide Hospital and involves 40 healthy individuals aged 18-65. Thirty random participants are receiving two doses of the vaccine three weeks apart; the remaining ten get a placebo.
 Professor Nikolai Petrovsky, of Vaxine and Flinders University, with the COVID-19 vaccine candidate. It was the first Australian-developed vaccine to enter phase 1 human trials, on July 1.
David Maruiz/AAP
Professor Nikolai Petrovsky, of Vaxine and Flinders University, with the COVID-19 vaccine candidate. It was the first Australian-developed vaccine to enter phase 1 human trials, on July 1.
David Maruiz/AAP
The primary purpose of a phase 1 trial is to show vaccine safety but the study will also allow a preliminary assessment to be made of its ability to induce an appropriate immune response against the SARS-CoV-2 virus.
Plans are already underway for phase 2 and 3 trials, which will likely involve recruitment of thousands of people in Australia and overseas, to confirm the effectiveness of the vaccine in preventing infection.
The aim is to complete the full clinical trial program by the end of 2020. Manufacturing scale-up is already underway with the aim to produce millions doses of vaccine, using multiple manufacturing sites around the world.
International
Oxford vaccine
Professor Katie Flanagan, University of Tasmania and Professor Magdalena Plebanski, RMIT
Researchers at the University of Oxford have just published encouraging results of their SARS-CoV-2 candidate vaccine called “ChAdOx1 nCoV-19”. It was tested in a joint phase 1 and 2 trial of 1,077 people aged 18-55.
This vaccine uses a chimpanzee virus called an adenovirus to carry SARS-CoV-2 genes into human cells, which then become factories for making the viral proteins. The body recognises the proteins as foreign, and mounts an immune response. Human adenoviruses circulate in the population and are known to be largely safe. Using a chimpanzee (rather than human) adenovirus has the advantage that the human system has not seen this virus before, so there is no pre-existing immunity that could clear out the vaccine before it can work.
The Oxford vaccine induced neutralising antibodies to the SARS-CoV-2 spike protein, as well as T cells, which might make it more effective. Some T cells can kill cells when the virus is hidden inside them and is replicating, and other T cells help B cells produce the neutralising antibodies, sustaining immune responses over time.
The good news is that neutralising antibodies were induced by two weeks after vaccination and lasted for at least 56 days. The vaccine also induced T cells to the spike protein of SARS-CoV-2. Importantly, the vaccine was safe over a 28-day monitoring period using standard safety assessments. The Oxford group previously found in a small study in monkeys that their vaccine induces anti-viral antibodies within similar timelines, and protects against SARS-CoV-2 infection by decreasing viral replication and damage in the lung in animals.
The encouraging results so far, together with other supportive data such as the monkey trials, have supported progression to multinational large-scale efficacy phase 3 trials to determine whether the vaccine protects against COVID-19 in humans. These further trials will also allow researchers to determine how different populations and subgroups, for example young and old adults, respond to this vaccine.
 University of Oxford scientists say early results suggest their COVID-19 vaccine is safe and can produce an immune response.
John Cairns/University of Oxford/AP/AAP
University of Oxford scientists say early results suggest their COVID-19 vaccine is safe and can produce an immune response.
John Cairns/University of Oxford/AP/AAP
CanSino vaccine
Professor Nigel McMillan, Griffith University
Chinese vaccine company CanSino Biologics has released its phase 2 clinical trial results for a vaccine to SARS-CoV-2, as reported in The Lancet. In the trial, 508 people were given two different doses of the vaccine and monitored for 28 days.
This vaccine uses adenovirus technology to deliver part of a SARS-CoV-2 protein to the immune system. The researchers reported good induction of B- and T-cell immunity, which are important for a strong overall immune response. They showed that the strong antibody responses were able to protect against virus infection in a lab animal model. Safety was good, with the usual and expected side effects of redness, fever, headache and swelling — although 9% of patients had a more serious fever and muscular or joint pain.
Overall, this is an important step forward in the vaccine development pipeline. The next phase will be to vaccinate larger groups of people to further examine safety, but more importantly this should be undertaken in areas with high virus load to test whether there is any protection against disease. The other important step will be to keep following these phase 2 patients to see how long the vaccine responses last as this will be critical in developing our long term response.
Correction: a previous version of this article stated CanSino’s vaccine had completed phase 1 trials. The company has in fact completed phase 2 trials. This has been corrected.
This article is supported by the Judith Neilson Institute for Journalism and Ideas.
Authors: Liam Petterson, Assistant Editor, Health + Medicine, The Conversation Australia




