In the evolutionary arms race between cane toads and lungworms, skin secretions play a surprising role
- Written by Martin Mayer, Postdoctoral Research Associate in Animal Ecology, Aarhus University
Unlike many other species of amphibians, the cane toad is thriving. It was introduced to Australia (and other places, such as Hawaii) to get rid of pest insects in sugar cane plantations. It had no effect on the pest insects, but soon after its introduction in 1935 it began to spread over large parts of the country.
And it didn’t come alone. Cane toads brought with them a parasite from their native range in South America, the lungworm nematode Rhabdias pseudosphaerocephala.
This invasion provides an ideal model to study the evolutionary “arms race” by which hosts and parasites adjust to each other, as we showed in a recent study.
How parasites can drive evolution
Parasites are the stuff of nightmares (just think of the creature in the movie Alien). Most people don’t think about parasites too much, and one reason is that over the past two centuries we humans managed to rid ourselves of most parasites that used to pester us.
Nonetheless, parasites are an essential part of most ecosystems and important drivers of evolution. But for most kinds of parasites, we don’t really know fundamental facts such as how they find their hosts in the first place and conversely, how hosts protect themselves from getting infected.
Read more: Meet Phronima, the barrel-riding parasite that inspired the movie Alien
The host and its parasites are locked in an “arms race” of adaptations and counter‐adaptations. Hosts evolve to detect and reject parasites; parasites evolve to deceive the host’s detection and suppression systems; then hosts evolve to defeat those new tricks, and so on.
This is why host–parasite interactions can be powerful drivers of evolution. Selection should favour hosts that can either reduce their chances of getting infected, which is called resistance, or limit the harm caused by a given parasite infection, which is called tolerance.
Cane toads vs lungworms
How does that arms race play out during a biological invasion, when both the host and the parasite are subject to powerful new evolutionary forces?
Using cane toads and their lungworm parasites, our new paper shows that the skin secretions of the cane toad host play a surprisingly important role.
The secretions that coat an amphibian consists of two parts: substances produced by the amphibian itself plus skin microbiome, mostly bacteria. These secretions contain many antimicrobial properties, which might help to fight off pathogens (such as chytrid fungus, the cause of so many amphibian declines).
At the same time, parasites try to overcome those barriers. Lungworm larvae (which develop in the faeces of an infected toad and then wait for a new toad to pass by) might use the smell of skin secretions as a cue to find their host in the first place.
We reasoned that the infective larvae of lungworms might even use the toad’s skin secretions to cloak themselves from the amphibians’ immune system when trying to make their way to the lungs (which is where they need to settle, mature, and reproduce).
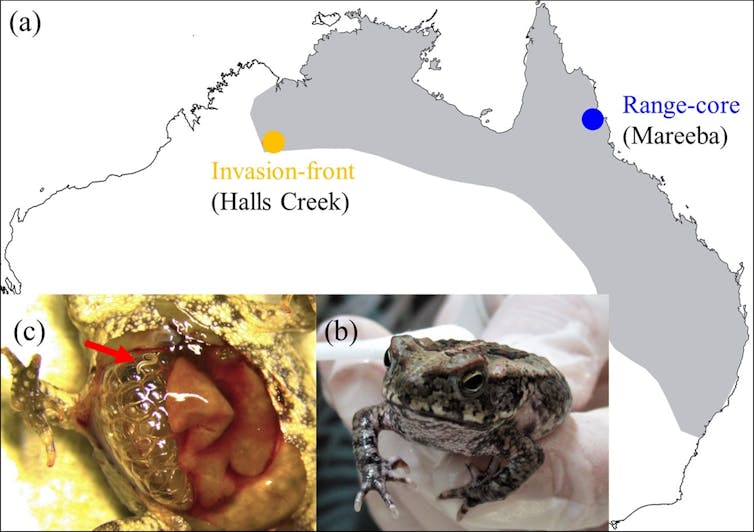 The cane toads’ current distribution in Australia (a), a cane toad used for our experiments (b) and a lungworm parasite located in a toads’ lung (c).
Mayer, Shine & Brown
The cane toads’ current distribution in Australia (a), a cane toad used for our experiments (b) and a lungworm parasite located in a toads’ lung (c).
Mayer, Shine & Brown
How the role of skin secretions changes
If hosts and parasites are constantly adapting to each other, we expect to see different strategies in different places for infection avoidance (in hosts) and host detection (in parasites). These differences might arise very quickly, such as during a biological invasion.
To test this idea, we experimentally infected cane toads from different regions in Australia with lungworm parasites from different regions. Additionally, we reduced skin secretions in some of the toads to test how their presence or absence affected the infection success of the parasite.
 A male cane toad in Northern Australia.
Martin Mayer, Author provided
A male cane toad in Northern Australia.
Martin Mayer, Author provided
We found that the role of skin secretions differed markedly between geographic regions.
In the toads’ core range (their main habitat) in tropical Queensland, toad skin secretions functioned as a cue for the parasite to find their host. But not only that, they also helped the parasites to infect the toads, meaning that more parasites managed to reach the toads’ lungs when skin secretions were intact. So it seems that these lungworms indeed cloak themselves from the host’s immune system.
But this was not the case at the toads’ invasion front (where toads are spreading into new territory) in Western Australia. Here, the skin secretions of cane toads appear to act as a defence against lungworms, reducing rather than enhancing their infection success.
Thus, although cane toads have been spreading through Australia for only 85 years, we see major divergences in the roles that their skin secretions play in host–parasite biology.
The state of the arms race
These geographical divergences fit well with the idea that cane toads in the core range have low resistance to parasite infection, because parasites are ubiquitous due to the nice warm and wet conditions year round.
Conversely, at the invasion‐front, where conditions are harsh and dry for most of the year, increased host resistance might be favoured – especially if parasite infection reduces the dispersal ability of a fast-moving invasion-front toad.
Thus, the cane toads on the invasion-front appear to be ahead in the arms race: they have adapted to the new conditions, while the lungworms are still catching up.
Authors: Martin Mayer, Postdoctoral Research Associate in Animal Ecology, Aarhus University




